Schedule I And Ii Narcotic Drugs
Controlled Substance Registration for Practitioner Frequently Asked Questions
Answer:This is a registration issued by the State of Connecticut, which is required for every practitioner who distributes, administers or dispenses any controlled substance or who proposes to engage in the distributing, prescribing, administering or dispensing of any controlled substance within this state. NOTE: This registration is required for Residents and Interns of Medicine.
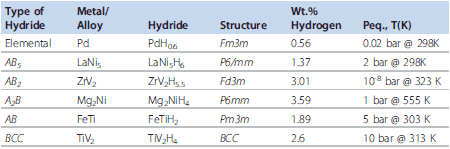
Schedule II drugs or other substance also have a high potential for abuse. They differ from schedule I drugs in that they do have a currently accepted medical use in treatment in the United States or a currently accepted medical use with severe restrictions.
Answer:
1. The applicant must be a practitioner, as defined by the Department of Public Health, as a physician, dentist, veterinarian, podiatrist, osteopath, optometrist, physician assistant, licensed pursuant to section 20-12b, as amended, advanced practice registered nurse as defined in subsection (b) of section 20-87a, nurse-midwife, scientific investigator or other person licensed registered or otherwise permitted to distribute, dispense, conduct research with respect to or to administer a controlled substance in the course of professional practice or research in this state. NOTE: According to the Department of Public Health, an Advanced Practice Registered Nurse (APRN) cannot order drugs, and a Physician Assistant cannot order drugs or dispense medications to a patient unless it is permitted in the Physician Assistants practice agreement.
Advanced Practice Registered Nurse (APRN)
Sec. 20-87a. Definitions. Scope of practice. (a) The practice of nursing by a registered nurse is defined as the process of diagnosing human responses to actual or potential health problems, providing supportive and restorative care, health counseling and teaching, case finding and referral, collaborating in the implementation of the total health care regimen, and executing the medical regimen under the direction of a licensed physician, dentist or advanced practice registered nurse.
(b) Advanced nursing practice is defined as the performance of advanced level nursing practice activities that, by virtue of post basic specialized education and experience, are appropriate to and may be performed by an advanced practice registered nurse. The advanced practice registered nurse performs acts of diagnosis and treatment of alterations in health status, as described in subsection (a) of this section, and shall collaborate with a physician licensed to practice medicine in this state. If practicing in (1) an institution licensed pursuant to subsection (a) of section 19a-491 as a hospital, residential care home, health care facility for the handicapped, nursing home, rest home, mental health facility, substance abuse treatment facility, infirmary operated by an educational institution for the care of students enrolled in, and faculty and staff of, such institution, or facility operated and maintained by any state agency and providing services for the prevention, diagnosis and treatment or care of human health conditions, or (2) an industrial health facility licensed pursuant to subsection (h) of section 31-374 which serves at least two thousand employees, or (3) a clinic operated by a state agency, municipality, or private nonprofit corporation, or (4) a clinic operated by any educational institution prescribed by regulations adopted pursuant to section 20-99a, the advanced practice registered nurse may, in collaboration with a physician licensed to practice medicine in this state, prescribe, dispense, and administer medical therapeutics and corrective measures. In all other settings, the advanced practice registered nurse may, in collaboration with a physician licensed to practice medicine in the state, prescribe and administer medical therapeutics and corrective measures and may request, sign for, receive and dispense drugs in the form of professional samples in accordance with sections 20-14c to 20-14e, inclusive, except that an advanced practice registered nurse licensed pursuant to section 20-94a and maintaining current certification from the American Association of Nurse Anesthetists who is prescribing and administrating medical therapeutics during surgery may only do so if the physician who is medically directing the prescriptive activity is physically present in the institution, clinic or other setting where the surgery is being performed. For purposes of this subsection, 'collaboration' means a mutually agreed upon relationship between an advanced practice registered nurse and a physician who is educated, trained or has relevant experience that is related to the work of such advanced practice registered nurse. The collaboration shall address a reasonable and appropriate level of consultation and referral, coverage for the patient in the absence of the advanced practice registered nurse, a method to review patient outcomes and a method of disclosure of the relationship to the patient. Relative to the exercise of prescriptive authority, the collaboration between an advanced practice registered nurse and a physician shall be in writing and shall address the level of schedule II and III controlled substances that the advanced practice registered nurse may prescribe and provide a method to review patient outcomes, including, but not limited to, the review of medical therapeutics, corrective measures, laboratory tests and other diagnostic procedures that the advanced practice registered nurse may prescribe, dispense and administer. An advanced practice registered nurse licensed under the provisions of this chapter may make the determination and pronouncement of death of a patient, provided the advanced practice registered nurse attests to such pronouncement on the certificate of death and signs the certificate of death no later than twenty-four hours after the pronouncement.
Physician Assistant
Sec. Free obd ii laptop software. 20-12d. Medical functions performed by physician assistants. Prescriptive authority.
(a) A physician assistant who has complied with the provisions of sections 20-12b and 20-12c may perform medical functions delegated by a supervising physician when: (1) The supervising physician is satisfied as to the ability and competency of the physician assistant; (2) such delegation is consistent with the health and welfare of the patient and in keeping with sound medical practice; and (3) such functions are performed under the oversight, control and direction of the supervising physician. The functions that may be performed under such delegation are those that are within the scope of the supervising physician's license, within the scope of such physician's competence as evidenced by such physician's postgraduate education, training and experience and within the normal scope of such physician's actual practice. Delegated functions shall be implemented in accordance with written protocols established by the supervising physician. All orders written by physician assistants shall be followed by the signature of the physician assistant and the printed name of the supervising physician. A physician assistant may, as delegated by the supervising physician within the scope of such physician's license, (A) prescribe and administer drugs, including controlled substances in schedule IV or V in all settings, (B) renew prescriptions for controlled substances in schedule II, III, IV or V in all settings, (C) prescribe and administer controlled substances in schedule II or III in all settings, provided in all cases where the physician assistant prescribes a controlled substance in schedule II or III, the physician under whose supervision the physician assistant is prescribing shall document such physician's approval of the order in the patient's medical record not later than one calendar day thereafter, and (D) prescribe and approve the use of durable medical equipment. The physician assistant may, as delegated by the supervising physician within the scope of such physician's license, request, sign for, receive and dispense drugs to patients, in the form of professional samples, as defined in section 20-14c, or when dispensing in an outpatient clinic as defined in the regulations of Connecticut state agencies and licensed pursuant to subsection (a) of section 19a-491 that operates on a not-for-profit basis, or when dispensing in a clinic operated by a state agency or municipality. Nothing in this subsection shall be construed to allow the physician assistant to request, sign for, receive or dispense any drug the physician assistant is not authorized under this subsection to prescribe.
2. The practitioner must be licensed or duly authorized to practice according to the appropriate state licensing board, commission or registration agency or, in the case of a hospital or other institution, by the appropriate state agency having jurisdiction over the licensure, registration or approval of such establishment.
3. The application is required to be completed and can be found at: http://www.ct.gov/dcp/cwp/view.asp?a=1622&q=446726 Bhajans by anuradha paudwal.
4. In order to maintain the registration current and active, the registration is required to be renewed every two years and expires on February 28 of every odd year.
Answer: A controlled (scheduled) drug is one whose use and distribution is closely monitored because of its abuse potential or risk. Controlled drugs are categorized in order of their abuse risk and placed in 'Schedules' by the federal Drug Enforcement Administration (DEA). Drugs with the highest abuse potential and no medical use are placed in Schedule I and those with the lowest abuse potential are in Schedule V. These schedules are commonly shown as C-I, C-II, C-III, C-IV, and C-V. Some examples of drugs in these Schedules are as follows:
Schedule I-drugs with a high abuse risk. These drugs have NO safe, accepted medical use in the United States. Some examples are heroin, LSD, Ecstasy, and mescaline.
Schedule II- drugs with a high abuse risk, but also have safe and accepted medical uses in the United States. These drugs can cause severe psychological and/or physical dependence. Schedule II drugs include certain narcotic, stimulant and depressant drugs. Some examples are morphine, cocaine, oxycodone (Percocet ®; Oxycontin®), hydrocodone with acetaminophen (Vicodin®), methylphenidate (Ritalin ®), and dextroamphetamine (Dexedrine ®). No refills of Schedule II medications are permitted.
Schedule III, IV, or V- drugs with an abuse risk less than Schedule II. These drugs also have safe and accepted medical uses in the United States. Schedule III, IV, or V drugs include those containing smaller amounts of certain narcotic and non-narcotic drugs, anti-anxiety drugs, tranquilizers, sedatives, stimulants, and non-narcotic analgesics. Some examples are acetaminophen with codeine (Tylenol® No. 3), diazepam (Valium®), alprazolam (Xanax®) and zolpidem (Ambien®). A maximum of five refills are permitted for Schedule III, IV, and V medications.
Schedule I And Ii Narcotics
Answer: The Controlled Substance Registration for Practitioners is a state registration that allows the prescriber to order, administer and dispense controlled substances to patients. The number is practitioner specific and is required regardless of practice location. Casio keyboard manuals pdf. The DEA registration number is a Federal identification number. In the State of Connecticut, the DEA requires the practitioner to obtain the Controlled Substance for Practitioner Registration prior to obtaining a new DEA number with a Connecticut address or changing the address of a current DEA to a Connecticut address. Please see this website www.deadiversion.usdoj.gov/ for more information about the DEA and to apply for a license.
Answer: If at the time that you are completing your application you do not have a specific practice address you can use your home address. You are responsible to update the address on your credential once you have a practice location(s). The updated information must be sent to the Department of Consumer Protection email address, at DCP.LicenseServices@ct.gov, within thirty (30) days. The practice site address is important because it permits a practitioner to store, administer and dispense controlled substances at a specific location. The Controlled Substance Registration for Practitioners is location specific.
Answer: Yes you can, however that may not necessarily expedite the process.
Class 2 Narcotics List
Answer: Practitioners who obtain this registration pursuant to the practitioner’s employment with a municipality, this state or the federal government shall not be required to pay the fee. If the practitioner works in a location secondary to the State or the federal government that is a private entity (i.e. moonlighting), they would no longer be fee exempt.
Answer:
The registrant must maintain effective controls against diversion of controlled substances into other than duly authorized legitimate medical, scientific, or commercial channels.
The registrant must only order, administer and dispense controlled substances for patients that they have a bona fide patient relationship with.
Compliance with all applicable state and federal laws and regulations concerning controlled substances.
The practitioner must maintain his/her federal DEA registration in good standing.
The practitioner must, at the time of application, have and maintain a professional license or certificate.
Must keep records of medical evaluations of patients and all controlled substances dispensed, administered or prescribed to patients by the practitioner.
Answer: If you have an active registration with the Department of Public Health it take approximately 15 business days for the approval of this registration from the time that you can see it as PENDING on our website www.elicense.ct.gov. Your registration CANNOT be processed until you have an active registration with the Department of Public Health.
Answer: The Controlled Substance Registration for Practitioners expires every two years on February 28th of odd numbered years. For the specific date of expiration please go to our website www.elicense.ct.gov and go to 'Lookup a License'.
Answer: Renewal notices will be emailed and/or mailed approximately 30 – 45 days prior to February 28th with the necessary information to renew online or through the mail. Please keep your email and address information current with our office to receive correspondence.
Answer: Please notify the Department of Consumer Protection Drug Control Division at DCP.DrugPractitioners@ct.gov with the date that you intend to stop practicing in Connecticut.
Answer: Yes. A practitioner may be required to obtain more than one Connecticut Controlled Substance Registration for Practitioners if they order and receive controlled substances at multiple locations. For instance, if the practitioner is a medical director of multiple nursing homes with controlled substance emergency boxes, the practitioner would be required to obtain a Connecticut Controlled Substance Registration for Practitioners for each nursing home, which maintains a controlled substance emergency box. In addition, the same is true for practitioners who order and receive controlled substances into multiple office practice sites.
Answer: No. Controlled Substance Registrations will be processed on a first come first serve basis in order to be fair to all applicants. Please allow up to 15 business days for approval of your registration provided you have an active license with the Department of Public Health in the State of Connecticut. Please track the process of your application on our website, www.elicense.ct.gov, as you will not receive your hard copy registration in the mail for an additional 7-10 business days.
Answer: The fastest way to change your address for the Connecticut Controlled Substance Registration for Practitioners is to send an email to DCP.LicenseServices@ct.gov. Please be sure to include your registration number, old address, new address and phone number in the email to assist us in processing your request in a timely manner. In addition please specify if your practice address is different from your mailing address.